Lab Overview

We have moved our lab 2 years ago to Weill Cornell to establish in vitro human and in vivo mouse models to understand how external signals control the internal signaling system that coordinates cell proliferation and movement. We are using cutting edge microscopy techniques, as well as genetic, pharmacological and systems biology approaches to attack the question how cells make these fundamental decisions.
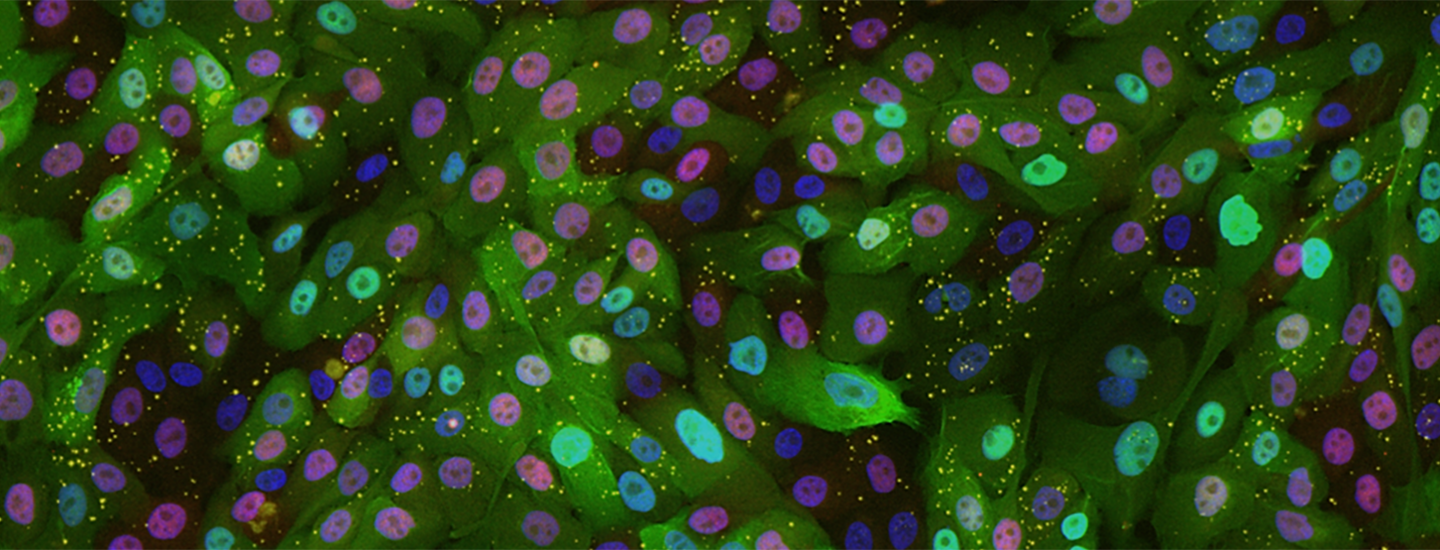
Open projects on cell proliferation: Ongoing control of cell proliferation is needed to maintain tissue function throughout life. Regulation of both cell-cycle entry and exit is important as most adult mammals have subsets of stem, progenitor and differentiated cells that can switch between proliferation and quiescence. The goal of our work is to understand the underlying signaling system how normal mammalian cells as well as cancer cells control cell-cycle entry and exit decision processes using cultured cell models, organoids, and in vivo single-cell analysis. This is an interesting problem in cell signaling as cells integrate tens of external hormones, growth factors, cell contacts as well as many internal stress and metabolic signals to ultimately make a single decision. We recently made a number of advances to understand this cell-cycle entry signaling system and have now critical techniques in place to provide definite answers to major open questions. A key part of all our projects is to develop new ways of studying proliferation and cell movement in parallel in vivo in mice and using micropatterned structures to develop 3D cell models that match physiological conditions.
See the following publications for more about this work:
- Suski JM, Ratnayeke N, … Meyer T*, Sicinski P*. CDC7-independent G1/S transition revealed by targeted protein degradation. Nature. 2022 May 4. doi: 10.1038/s41586-022-04698-x (*corresponding authors)
- Cappell SD, Mark KG, Garbett D, Pack LR, Rape M, Meyer T. 2018. EMI1 switches from being a substrate to an inhibitor of APC/CCDH1 to start the cell cycle. Nature. 558, 313-317.
- Yang HW, Chung M, Kudo T, Meyer T. 2017. Competing memories of mitogen and p53 signalling control cell-cycle entry. Nature. 549, 404-408.
- Spencer SL, Cappell SD, Tsai FC, Overton KW, Wang CL, Meyer T. 2013. The Proliferation-Quiescence Decision Is Controlled by a Bifurcation in CDK2 Activity at Mitotic Exit. Cell. 155, 369-83.
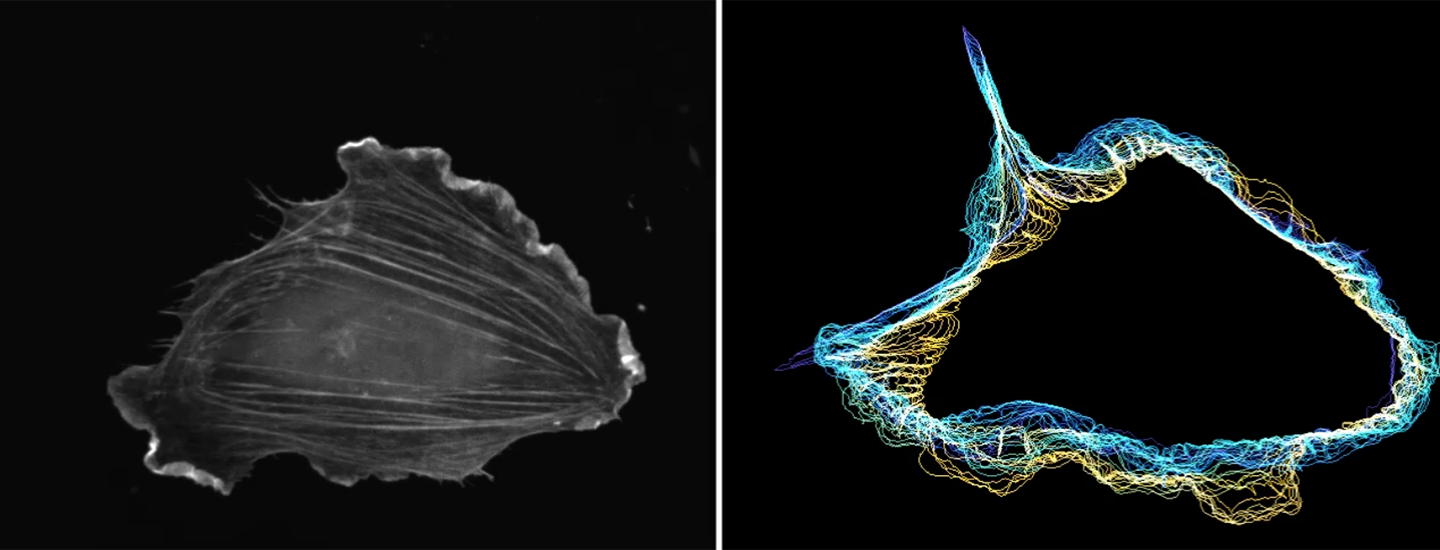
Open projects on cell movement: An open question in the control of cell movement is how cells sense contact with other cells and then transduce these signals to control not only movement but also proliferation. We are particularly excited about our striking recent findings of the role of directed signaling and movement by the polarized distribution of cortical actin, membrane curvature, ER-plasma membrane contacts, actin bundling proteins, as well as growth factor receptor signals.
See the following publications for more about this work:
- Bisaria A, Hayer A, Garbett D, Cohen D, Meyer T. 2020. Membrane proximal F-actin restricts local membrane protrusions and directs cell migration. Science Jun 12 DOI: 10.1126/science.aay7794
- Hayer A, Shao L, Chung M, Jouber LM, Yang HW, Tsai FC, Bisaria A, Betzig E, Meyer T. 2016. Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells. Nat Cell Biol. 18, 1311-1323.
- Yang HW, Collins SR, Meyer T. 2016. Locally excitable Cdc42 signals steer cells during chemotaxis. Nat Cell Biol. 18, 191-201.
- Yang HW, Collins SR, Meyer T. 2016. Locally excitable Cdc42 signals steer cells during chemotaxis. Nat Cell Biol. 18, 191-201.
- Winans AM, Collins SR, Meyer T. 2016. Waves of actin and microtubule polymerization drive microtubule-based transport and neurite growth before single axon formation. Elife. 5, e12387.
- Tsai FC, Seki A, Yang HW, Hayer A, Carrasco S, Malmersjö S, Meyer T. 2014. A polarized Ca2+, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nat Cell Biol. 16, 133-44.
- Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. 2013. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 339, 1445-8.
- Wollman R, Meyer T. 2012. Coordinated oscillations in cortical actin and Ca(2+) correlate with cycles of vesicle secretion. Nat Cell Biol. 14, 1261-9.
- Galic M, Jeong S, Tsai FC, Joubert LM, Wu YI, Hahn KM, Cui Y, Meyer T. 2012. External push and internal pull forces recruit curvature-sensing N-BAR domain proteins to the plasma membrane. Nat Cell Biol. 14, 874-81.


